A DEMONSTRABLE LINE ON EXTRACTED TEETH INDICATING THE LOCATION OF THE OUTER BORDER OF THE EPITHELIAL ATTACHMENT
|
Charles C. Bass, M.D.
School of Medicine, Tulane University of Louisiana,
New Orleans, La.
Vol. 25, No. 6, October 1946
|
|
The line to which attention is here directed may be seen on almost any extracted tooth specimen, when prepared and examined by appropriate methods. It consists of a narrow zone along which the border of the cuticle, which is laid down by the epithelial attachment, is disintegrating and stains heavier than the normal cuticle. Although this line must have been seen previously by others, I have not found any published mention or reference indicating that it has been recognized.
The epithelial attachment was first recognized and described by Gottlieb1 in 1921. Since that time it has come to be generally accepted in scientific dental literature and descriptions are to be found in most of the later textbooks embracing the histology and pathology of the periodontal tissues2,3,4,5,6.
When the eruption of a tooth is complete and it has reached the normal occlusal level, the epithelial attachment consists of a band of epithelial tissue, from one to several cells thick, attached to the enamel of the rootward third or half of the anatomical crown. It is derived from, and consists of, a remaining portion of the enamel organ. At this time it extends from the gingival crevice to the cementoenamel junction.
As the tip of the erupting tooth pushes its way through the outer enamel epithelium and the oral epithelium, these epithelial structures grow together1,3,6. Thereafter the epithelial attachment, as long as it remains entirely on the enamel, consists of this band of reduced enamel epithelium, to the outer surface of which the oral epithelium is organically attached.
As the location of the epithelial attachment moves towards the apex, its width becomes narrower and narrower. Whereas, when it is first established entirely on the enamel its width is equal to from one third to one half the length of the anatomical crown, when it is far down on the cementum it is only a fraction as wide. However, at any stage of progress of the usual pathological exfoliation (periodontoclasia) there is always a band, wider or narrower, of epithelial tissue (epithelial attachment) surrounding the tooth. The outer border of the epithelial attachment marks exactly the extent to which the soft tissue attached to the tooth has receded at any place. The demonstable line to which attention is called, is always located immediately adjacent to the outer border of the epithelial attachment. Therefore it accurately indicates the location of the important tissue and may be found useful in studying extracted teeth relative to periodontoclasia.
|
|
MATERIAL
Specimens may be examined soon after they are extracted; however, it is usually more convenient and more satisfactory to collect teeth, preserve them in formalin (2.5% sol.) and to examine them as desired later. If the formalin solution is changed occasionally, specimens may be kept for long periods of time and will be entirely satisfactory for study when needed. I have kept teeth preserved in this way for more than 3 years and they are still satisfactory for study in relation to both caries and periodontoclasia.
Quantities of teeth from extraction clinics or private practice may be collected and preserved in this way, for one's own studies, and for instruction and demonstration to students and others. It should be mentioned that specimens that have been stained and examined, and found interesting and instructive, can be preserved in the formalin solution for demonstration later to others.
EQUIPMENT
For satisfactory microscopic study of extracted teeth for any purpose one requires a good dissecting microscope and some facility for illuminating the object from above. I use a Zeiss stereoscopic dissecting microscope (XV) with inclined eyepieces and built-in illumination for incident light. Other means of lighting the object from above can be devised, a small spotlight being better than wide field lighting. The Nicholas illuminator (No. 31-33-49-01, Bausch & Lomb) should be almost as satisfactory for this purpose as the built-in light.
For routine work a magnification of about X 12 is referable. I use routinely 2 X objectives and 6X oculars. Although several other and higher magnifications are available, I find that I use them only occasionally and then for some special purpose.
Specimens are kept wet while they are being examined under the microscope. For this purpose a shallow glass dish, with a little water in it, is placed on the stage of the microscope. the top section of a Petri dish, outside measurements 10 cm. X 5 cm., is just right for this purpose.
|
The best instrument I have used for holding and manipulating teeth under the microscope is Rochester Pean forceps No. 522 1/4. These can be obtained from dealers in surgical instruments. A longitudinal groove cut in the grasping end of the blades greatly improves this instrument for holding the root end and manipulating tooth specimens.2
For handling specimens, transferring to and from staining solutions, etc., a pair of ordinary surgeon's thumb forceps, 7 1/2” size, is needed.
For teasing out, removing and manipulating very small particles, fine teasing needles and micro-instruments are required. These are made from ordinary fine gauge (No. 10) silver steel sewing needles which are driven (eye-end) into 4” lengths of 3/16” wooden doweling, and then shaped and sharpened on a fine grain stone (Arkansas pocket stone No. AP 12). In manipulating small particles of cuticle and other material, such as is contemplated in this paper, I find that I use mostly a needle whetted to a spade or chisel shape and about 0.35 mm wide. Round long tapered needles with thin sharp points are also needed.
Some such instrument as the S. S. White explorer, single ended, No.7, is sometimes useful in microscopic study of the area and tissues under consideration here.
PREPARING SPECIMENS FOR MICROSCOPIC EXAMINATION
Many different stains and staining methods may be used for preparing extracted teeth for microscopic study; some better for one purpose and others better for other purposes. Workers may employ stains with which they are accustomed to working, but for the demonstration of this line crystal violet will be found most satisfactory.
Our first objective is to see the line under consideration and learn to recognize it on different teeth. For this purpose choose several teeth, preferably specimens on which the outer border of the epithelial attachment had not moved beneath the cemento-enamel junction and therefore was still located on the enamel. These should be cleaned off by brushing them gently and washing them in running water. An ordinary toothbrush will serve the purpose but it is better to use a very soft brush, such as a child's size brush with nylon bristling about 0.18 mm in diameter, instead of the heavier adult size with bristles twice as thick. Very hard stiff bristles are objectionable. A little soap on the brush helps to clean off the tooth. This brushing removes most of the soft bacterial film, debris and other loose material which would be somewhat in the way of our present purpose of showing up the line most clearly. Where there are large pieces of gingiva and other soft tissue attached to the tooth, these should be trimmed away (Bard-Parker knife, No. 11 blade). This can be done better with the specimen held under the dissecting microscope.
Next place the specimens in the crystal violet solution (0.5% in water) in a small, low form, widemouth bottle or other container. Allow to stain from 1 to 5 minutes, remove, brush again, wash in running water and examine. Keep the specimen (held by the root with Pean forceps) wet while examining it, by turning it over from time to time in the water in the dish.
|
RECOGNIZING THE LINE
A purple stained line on the tooth will be found readily (figs. 1-7), its location and course varying according to the rootward progress of the outer border of the epithelial attachment at any particular place. On some specimens the line can be found only in separate places, on others it may be traced all the way around the tooth. At first, instead of expecting to recognize the line all the way around a given specimen, it is better to examine several different specimens until one becomes familiar with the line and learns not to confuse it with other things on the tooth.
Abbreviations used:
zdeac- zone of disintegrating epithelial-attachment cuticle designatng the demonstrable line which is the subject of this paper;
eae- epithelial-attachment cuticle below the disintegrating zone;
ec- enamel cuticle;
cej- cemento-enamel junction;
pm- fibers of the torn periodontal membrane remaining attached to the cementum of the extracted tooth; in this paper we are interested only in the outer border of this membrane in relation to the zdeac;
ea- epithelial-attachment.
|
 |
| Figs. 1,2,3,4 |
|
|
|
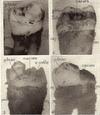
Fig. 1. Third molar; zdeac on enamel; note higher location on distal than mesial side; pm about at ccj.
Fig. 2. Approximal side, second molar; zdeac on enamel; note how it dips below contact point where caries has already occurred; some of en. cells on eac; pm at cej.
Fig. 3. Badly decayed crown but pm still at cej; zdeac on enamel and in places near to caries.
Fig. 4. Incompletely erupted 3rd molar; caries of exposed part of crown; zdeac on enamel passes over un-erupted cusp.
|
Next, one should prepare and examine in the same way, a number of specimens in which the soft tissues have receded, in some places at least, well below the cemento-enamel junction. Include some in which more than half of the root has been exposed. The purple stained line on such specimens will be found even more easily and satisfactorily (figs. 8-17). Its course varies greatly. At one place it runs crosswise of the root, at others diagonally, or even up and down (figs. 15-17). In most places its course conforms to the course of the nearby border of the hard scale and calculus found on such specimens (figs. 5, 9,10, 11, 13, 14, 15, 16, 17, 18, 21, 22, 24, 25).
|
 |
| Figs. 5,6,7,8 |
|
|
Fig. 5. Zdeac on enamel dips below calculus just below cavity; pm has receded below cej.
Fig. 6. Heavy zdeac on enamel on short, badly decayed molar; pm has receded to below (faintly shown) cej at most places.
Fig. 7. Zdeac on enamel of deciduous tooth just below worn approximal contact point.
Fig. 8. Zdeac on enamel at left, extending across and below cej, right.
|
|
It is impressive to see this line standing out so clearly on specimens on which the soft tissues had receded until only a small portion was still attached. It is present about the apex, even up to the last stages of exfoliation (fig. 17).
RELATION OF EPITHELIAL ATTACHMENT TO THE LINE
Having learned to recognize this line wherever it is present, one is now ready to proceed to study its relation to the epithelial attachment and to learn more of the nature of the line itself. Different procedures are available and selection will have to be determined by the circumstances and by the equipment and technical skill at the worker's command.
When a tooth is extracted, usually a few, if not many, of the cells of the epithelial attachment are torn from the soft tissue and remain attached to the tooth. Some of those at and near the outer border especially remain on the tooth. Usually most of the cells are removed by the brushing which was suggested above and intended to help bring out the line clearly. Therefore it is desirable now to prepare selected specimens in the same way except omitting the brushing. Work first with specimens on which the outer border of the epithelial attachment is located on the enamel.
|
 |
| Figs. 9.10.11.12 |
|
|
|
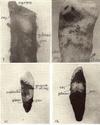
Fig. 9. Zdeac on cementum of root from which most of crown has been lost from decay line takes course around heavy lump of calculus which was broken off in extracting; note fairly uniform width of eac between zdeac and pm.
Fig. 10. Zdeac far down on cementum; course conforms to lower border of scales of calculus which remained after brushing.
F1:g. 11. Zdeac far down on cementum dips and conforms somewhat to calculus above.
Fig. 12. Zdeac on cementum; cej can be seen at one place left; part of calculus was broken off; note higher point, of pm corresponding to course of zdeac.
|
Notwithstanding the presence of more or less overhanging loose debris and bacterial film, one who already has become familiar with the deeper staining line will have no difficulty in recognizing it. At favorable locations one may find areas or patches of raised, heavier stained material on the tooth just below the line, and between it and the adhering portions of the periodontal membrane (figs. 2, 3, 5). This proves later to be epithelial cells attached to the tooth. After a little experience one is able to recognize these attached cells, even under the low magnification of the dissecting microscope.
|
 |
| Figs. 13,14,15,16 |
|
|
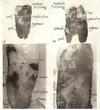
Fig. 13. Zdeac far down on cementum conforms, in general, to course of nearby border of calculus; cej shown sharply.
Fig. 14. Zdeac running crosswise or up and down around scales of calculus extending more than 1/2 length of root.
Fig. 15. Zdeac running crosswise or up and down around scales of calculus extending more than 1/2 length of root.
Fig. 16. Zdeac located just below cej at right, extends downward across, then curves back and down towards the apex; some of pm bas been lost in preparation; calculus and plaque material on left correspond closely to course or zdeac.
|
For the purpose of seeing and differentiating these cells on the tooth one requires some facility for illuminating the object from above while examining it with suitable magnification under the regular microscope. I use a Bausch & Lomb surface illuminator (31-33-05-02) which can be used with objectives up to 16 mm. However, one can improvise satisfactory surface lighting for use with objectives which have working distances of 15 mm. or more, simply by directing the light onto the object from some kind of lamps properly placed, one on either side of the microscope. A 6X objective (working distance 15.5) can be used in this way and with appropriate eyepieces gives sufficient magnification for recognition and differentiation of epithelial cells on the surface of teeth. For such examination the specimen is placed in a Petri dish containing just enough water to cover the tooth.
|
 |
| Figs. 17,18,19,20 |
|
|
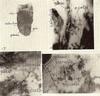
Fig. 17. Zdeac running diagonally across near apex; tooth held in by only small amount of epithelial attachment and pm as final exfoliation approaches.
Fig. 18. Area on enamel crown taken with surface illuminator, original mag. X27; zdeac with calculus just above and some epithelial cells still attached to the eac below approximate measurements -edge of calculus to zdeac 10 microns, zdeac 20 microns wide, zdeac to pm 50 microns.
Fig. 19. Area on enamel crown taken with surface illuminator, original mag. X72; zdeac above, some attached epithelial cells and on enamel lamella (or crack) below.
Fig. 20. Area on enamel crown taken with surface illuminator, original mag. X72; edge of cavity, upper left corner; zdeac faintly below to left; epithelial cells attached to eac running right up to lower side of zdeac; one heavily stained lamella (or crack).
|
The crystal violet stain does not give as sharp differentiation of nucleus and cytoplasm as many other stains, especially such strong nuclear stains as hematoxylin. Although crystal violet will be found satisfactory for most purposes, it is suggested that those who wish other effects try 1) hematoxylin and eosin, 2) dilute Giemsa, or 3) fuchsin and toluidine blue (0.05% basic fuchsin in N/500 HCl, 3 minutes, wash, toluidine blue 1% in 20 per cent alcohol 1/2 to 1 minute, wash).
|
 |
| Figs. 21,22,23,24 |
|
|
|
Fig. 21. Area on cementum, taken with 2 reflector lamps, original mag. X18; zdeac curves around calculus scale; fairly uniform distance from zdeac to pm.
Fig. 22. Area far down on cementum, original mag. X4; zdeac runs irregularly across root, conforming somewhat to nearby border of calculus scales; pm partly torn away at left.
Fig. 23. Area on crown of deciduous tooth (see fig. 7) taken with surface illuminator, original mag. X27; sharp line, zdeac, left after brushing which removed outer border of the zone and some plaque material, leaving almost clear space between; one lamella (or crack) and eac extend far down to pm located at cej.
Fig. 24. Area on crown just. above cej, taken with surface illuminator, original mag. X27; zdeac has scales of calculus projecting into it; indicates how calculus crowds down eae in some places.
|
|
RELATION OF EPITHELIAL CELLS TO DENSER STAINED LINE
Examining specimens in this way the relation of the outer border of the epithelial attachment to the denser stained line, as indicated by the attached cells, can be recognized. The cells are flattened and spread out towards the lower side of the purple line (fig. 18). Sometimes the layer of cells at the border is only 1 or 2 cells thick. It may be several cells thick farther down. These flat epithelial cells appear to spread out to cover the surface and to be connected by projections from their reticulated cytoplasm (figs. 19, 20). Often mitosis can be seen, showing nuclei in different stages of division and probably indicating the active reproduction that must be required at this place where there is considerable loss of cells going on continuously.
EXAMINATION OF PIECES OF CUTICLE REMOVED FROM TOOTH
The relation of the outer border of the epithelial attachment to the line, as indicated by the adhering cells, and further information about the nature of the line itself can be studied better by an entirely different procedure, not requiring surface illumination for the regular microscope, but requiring somewhat more delicate technic. It consists of removing a suitable, selected piece of cuticle, including both adhering epithelial cells and the line, and restaining. When properly stained and mounted, such a preparation can be examined satisfactorily under the higher powers of the microscope, including oil-immersion objectives. Not only can the attached cells be seen and differentiated but the nature and composition of the line can be seen clearly.
Since the technic here required includes steps similar to some that are necessary in other studies of extracted teeth, it will be given in some detail. This is done, however, with the full realization that others may prefer to develop and employ methods which seem more appropriate and are more convenient for them. Select a stained specimen on which the line and some epithelial cells are located on the lower half of the anatomical crown. Under the dissecting microscope, with the point of a sharp knife (Bard-Parker, No. 11 blade) make cuts through the cuticle outlining the area selected to be removed. Include in this window or piece of cuticle to be removed, some of the attached cells and the full width of the stained line. It is better to go just a little beyond it; however, for present purposes, the much thicker bacterial material above the line should be avoided.
In some places, at least, the lines that have been cut through the cuticle can be seen under the dissecting microscope. One can select and cut in the same way, outlines of other areas which it is desired to remove and examine. Small pieces are much easier to manipulate and mount. Pieces less than 1 mm wide and 2 or 3 mm long should be used at first. Later one may wish to mount larger pieces but the manipulations become more difficult and the results are less satisfactory. Having cut around, with the sharp pointed blade, one or more areas for removal, the cuticle is loosened by immersing the tooth in hydrochloric acid solution. (For all routine work I use HCl 10 cc., formalin 5 cc., water 85 cc. Other weaker solutions of HCl are quite satisfactory. One per cent HCl can be used but its action is slower and weaker). The decalcification is carried only far enough to release the cuticle and preferably not far enough to dissolve the entire thickness of enamel, in which case the floating cuticle is more difficult to recover and handle for the present purpose. The cuticle is released more quickly on some teeth than others, but usually 2 to 5 minutes in the acid is long enough.
After the proper time in the acid, the tooth is removed with forceps, grasped by the root with the modified Rochester Pean forceps and transferred (without washing) to the dish of water on the stage of the dissecting microscope. The specimen must be handled gently and carefully, at this stage, to avoid dislodging and losing the piece of material wanted. With a teasing needle the piece desired is teased loose from any connection (if any) to the surrounding membrane and dropped into the shallow water in the dish. From there it can be transferred with the flattened (0.35 mm) needle, used as a section lifter, to a drop of water on slide (or into another dish of water if several pieces are to be used) to wash out the acid. A minute or two is all that is necessary, especially if the specimen is transferred to, and washed in, a second separate drop of water on the slide.
The specimen which has lost most of the first stain in the acid is now ready for restaining. Anyone of many different stains and staining methods may be employed. Fuchsin and especially carbolfuchsin is a strong stain for this cuticle. One should first look at specimens stained with it alone. It stains, not only the cuticle upon which the epithelial cells rest but even better the disintegrating border of this cuticle which constitutes our line. The much thinner layer of cuticle just above the line is also stained, more or less. Epithelial cells, both nucleus and cytoplasm, are stained intensively but can be recognized without difficulty. Our main objective here is merely to recognize and identify the cells from the epithelial attachment which remained attached to the cuticle and to observe the relation of the outer border of these cells to our line.
A convenient procedure is to put a drop of 50% glycerin (glycerin 50 cc., water 50 cc.) on a slide near one end. Add to it a small drop of carbolfuchsin. Mix with a needle and transfer the specimen into this diluted stain. Staining proceeds rapidly. One or 2 minutes is sufficient for most purposes. However,
staining may be continued much longer and thereby stain more deeply, the almost transparent normal cuticle. Place a drop of 50% glycerin on the slide (or on a separate one if desired) and near it a smaller droplet in which the specimen is to be finally mounted for examination. Transfer the specimen to the large drop of glycerin and wash it, moving it about with the needle to hasten the process. Then transfer the specimen to the smaller droplet of glycerin. Spread it out and orient it by the use of teasing needles, one in each hand. Put on a cover glass.
There are advantages here in using smaller than the usual size of cover glasses. It is convenient to cut the regular 7/8” square size into 4 square pieces, with the aid of a glass marking pencil or scriber. A pair of medium point, curved forceps (Fischer No. 8-882) is useful in placing the small cover glass on the specimen. The cover glass is pressed down and any excess of glycerin is blotted away with the corner or edge of a towel.
Better effects for some purposes may be obtained by adding a small drop of crystal violet to the diluted carbolfuchsin on the slide. The disintegration of the cuticle and its separate layers may be seen sometimes better with this double stain. Anyone of many other stains may be used including hematoxylin and eosin, Giemsa or the fuchsin and toluidin blue mentioned above.
|
COMPOSITION OF LINE
The line is seen to consist of a heavier stained zone or border of the same membrane on which the epithelial cells are attached. It consists of 2 or more layers which are broken into irregular patches, especially at the ragged outer border. At the inner border the heavier stained membrane merges into and is continuous with the normal cuticle to which the epithelial cells are attached. Places may be found where irregularly shaped gaps or spaces seem to be broken out of one layer, leaving other layers showing more clearly (figs. 26, 27, 28).
|
 |
| Figs. 25,26,27,28 |
|
|
|
Fig. 25. Area takes in cej running diagonally across, taken with surface illuminator original mag. X27; zdeac runs along cej at left; along middle it is stained heavily and located well above cej; scale of calculus just above; several enamel lamellae (or cracks) in part covered with eac, one in enamel from which eac has been destroyed.
Pig. 26. Piece of cuticle released by acid and teased from suda,ce of enamel, original mag. X72; zdeac very ragged and darker stained than eac below; at least 2 or 3 layers of the latter can be made out but still more layers of the disintegrating zone can be seen.
Fig. 27. Piece of cuticle from enamel, original mag. X72; zdeac appears to have several layers; at least 2 layers of eac below; piece of plaque material upon ec which is continuous with and underlies eac.
Fig. 28. Piece of cuticle from enamel, original mag. X72; zdeac and eac below from which patches of one or more layers are torn out; piece of plague material upon ec and another piece at left folded down over the zdeac.
|
In addition to the larger breaks and cracks seen in the outer part of this membrane, upon high magnification and appropriate adjustments of the light one sees that the material is checkered and breaking up into still smaller divisions. Perhaps it can be spoken of as "crinkled" (figs. 31, 31).
Pieces loosened on one side and attached On the other may be seen. It is very evident that the cuticular material here is breaking up or disintegrating, and as a result stains heavier than the other part of the cuticle (fig. 29). The disintegration accounts for the heavier staining. Our line therefore consists of a portion or zone of the cuticle which is in different stages of disintegration.
On well made preparations we find that one layer, usually extremely thin, extends (occlusalward) beyond the line or zone and is actually continuous with the thin enamel cuticle which covers the entire crown ("primary enamel cuticle")(7, 8). Apparently the cuticle which disintegrates is superimposed upon, and attached to, this deeper layer which rests upon the enamel. On a favorable specimen and by delicate technic I have been able to make out at least 5 different layers or lamina of cuticle at the disintegrating zone, and I am inclined to believe a still larger number may be present in some specimens.
|
Fig. 29. Piece of cuticle from enamel, original mag. X72; breaking up and multiple layers at this area, emphasized by small condenser diaphragm opening and greater depth of focus; eac below is seen to consist of at least 2 or 3 layers; lower, left of center; a piece of this extremely thin cuticle is folded sharply back and to the side.
Fig. 30. Cuticle from enamel, field near rootward edge of zdeac, original mag. X810; early stage of disintegration, mottled or crinkled appearance before any breaking apart has occurred.
Fig. 31. Cuticle from enamel, field a little nearer edge of zdeac than fig. 30, original mag. X810; very fine mottling or crinkling, early stage of disintegration at this area.
|
|
Still further light is thrown upon the cuticle by scratching and tearing it before removing it for higher magnification. With the teasing needle one can make scratches across the heavier stained zone and actually scrape back (or off) all except the deepest, extermely thin layer which rests upon the enamel. A specimen obtained by cutting a window, as described above, including the area which was scratched, if successfully prepared, will show this deepest layer to be continuous, whereas the outer heavier stained layers have been scrape off from it.
ATTACHMENT CELLS AND LINE ON CEMENTUM
As the epithelial attachment moves towards the apex it crosses over the cemento-enamel junction and at a time rests partly upon enamel and partly upon the cementum below the junction line (figs. 8, 25). As it moves on down farther the outer border passes the cemento-enamel junction and the attachment then rests entirely upon cementum.
Gottlieb pointed out that a cuticle-like membrane underlies the epithelial attachment and, in fact, is the means by which the cells of the attachment are attached to the cementum. This cuticle is laid down or produced by the epithelial attachment and was called by him cuticula dentis, or dental cuticle, as distinguished from the (so called) primary (and the secondary enamel) cuticle.
This dental cuticle on the cementum beneath the epithelial attachment can be seen in sections which include the root and soft tissues. It is now generally recognized and accepted (2,4,5, 6).
Often there are some cells from the epithelial attachment attached to the cuticle upon the cementum. We can find some of these extending near to the lower side of the heavier stained zone of disintegrating cuticle, which is our line, just as we did when the line was located on the enamel.
The disintegrating cuticle producing the line and the normal cuticle with or without attached epithelial cells can be scraped off in the same way as when they are located on the enamel but there is no underlying primary enamel cuticle. Neither can we release the cuticle with acid as when it is located on the enamel. The acid does not cause complete loss of cementum, as it does enamel, and thereby free the cuticle. Therefore the cuticle remains firmly attached to the cementum, although the latter may be completely decalcified.
The epithelial cells can be differentiated and the nature of the line on the cementum can be observed directly upon the tooth by suitable magnification under the regular microscope, as described above for specimens wherein they are located on the enamel. It is also possible to tease off very small particles of this membrane with cells attached to it, or the line itself, and to stain, mount, and examine them under higher magnifications. Although such preparations are less satisfactory than those from enamel surfaces, it is quite possible to recognize some of the epithelial cells and to see that the line consists of the same kind of heavier stained, disintegrating, laminated, cuticular membrane.
|
NAME SUGGESTED FOR CUTICLE AND THIS LINE
This cuticle, the disintegrating border of which produces the line to which we direct attention, underlies the epithelial attachment, is laid down or produced by the epithelial attachment and attaches the cells of this tissue to the tooth, either on the enamel or on the cementum. Therefore I propose to call it the epithelial-attachment cuticle; and this demonstrable line, the zone of disintegrating epithelial-attachment cuticle or zdeac.
Those who do not accept this term may prefer to call this line the zone of disintegrating secondary cuticle or the zone of disintegrating dental cuticle, in conformity with names applied to this cuticle by Gottlieb. Or, if one adopts the name for the cuticle suggested by Glickman and Bibby (9) he may prefer to call it the zone of disintegrating transposed-crevicular cuticle. Whatever name is accepted it should be clearly understood that the demonstrable line to which attention is here directed, consists of a narrow zone (or band) along which the cuticular membrane which is always present beneath the epithelial attachment, whatever the location of the latter on the tooth, is breaking down or disintegrating; and further that this cuticle, when located on the enamel overlies, is attached to, but is different from, the so-called primary enamel cuticle (fig. 23).
It should also be understood that whether there are many, few or no cells from the epithelial attachment found on a given specimen, the deeper stained line exactly conforms to, and indicates the location of, the outer border of the epithelial attachment before the tooth was extracted. After one has learned from sufficient experience that the cells of the attachment come right up to, but do not overlap this line, it serves as a useful landmark indicating exactly the extent of recession of this tissue at any particular place.
|
SUMMARY
1. Attention has been directed to a demonstrable line on extracted teeth which is a good guide to the location of the outer border of the epithelial attachment. Technic for demonstrating it was given.
2. The line consists of a denser stained border or zone of the cuticular structure upon which the epithelial attachment rests. The denser staining is due to disintegration of this part of the cuticle.
3. It is proposed to call the cuticle upon which the epithelial attachment rests and which is produced by the epithelial attachment, the epithelial-attachment cuticle and this line, the zone of disintegrating epithelial-attachment cuticle or zdeac.
REFERENCES
1. GOTTLIEB, B., Del' Epithelansantz am Zahn. Disch. Monalschr. f. Zahnheilk., 39: 142-147, 1921.
2. ORBAN, B., Oral Histology and Embryology (St. Louis, Mosby, 1944).
3. CHURCHILL, H. R., Meyer's Normal Histology and Histogenesis of the Human Teeth and Associated Parts. (Philadelphia, J. B. Lippincott Co., 1935.)
4. GOTTLIEB, B., ORBAN, B., AND DIAMOND, M., Biology and Pathology of the Tooth and Its Supporting Mechanism. (New York, Macmillan Co., 1938.)
5. NOYES, F. B., SHOUR, I., AND NOYES, H. J., Dental Histology and Embryology. (Philadelphia. Lea and Febiger' 1943.)
6. KRONFELD, R., Histopathology of the Teeth, 2nd ed. (Philadelphia, Lea and Febiger, 1939.)
7. KRONFELD, R., The Epithelial attachment and So-called Nasmyth's Membrane. J. A. D. A., 17: 1889-1907, 1930.
8. VALLOTON, C. F. An acquired pigmented pellicle of the enamel surface, Review of literature. J. D. Res., 24: 161-169, 1945. (This includes a review of the literature relative to the enamel cuticle. The list of references given will be found convenient for those interested in the subject.)
GLICKMAN, 1., AND BIBBY, B., The Existence of Cuticular Structures on Human Teeth. D. Res., 22: 91--96,1943.
|
by
Charles C. Bass, M.D.
School of Medicine, Tulane University of Louisiana,
New Orleans, La.
|
|
|